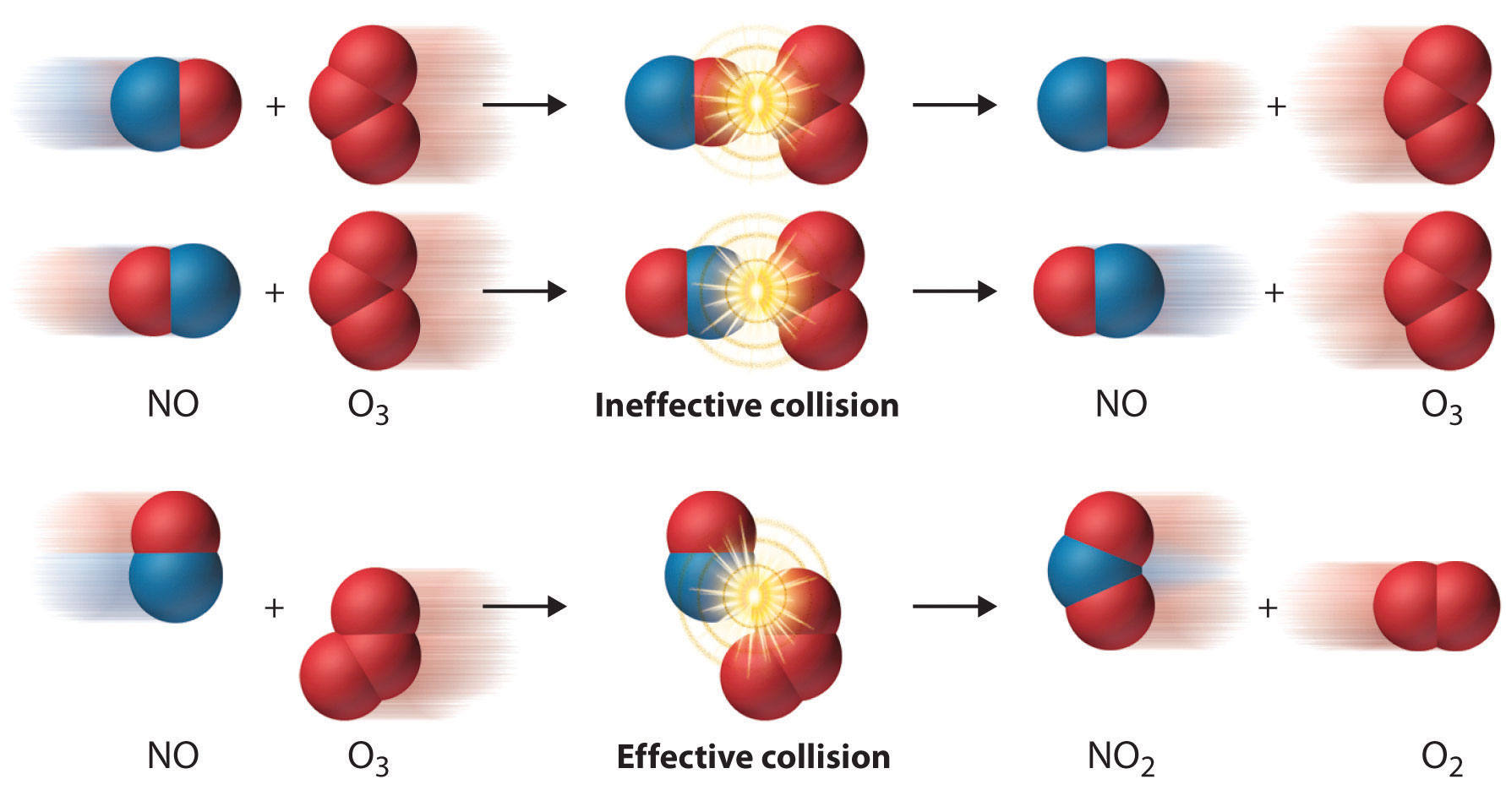
Threshold Energy In Chemical Kinetics . The minimum amount of energy required by the colliding molecules to yield the products is called threshold energy (et). Mx + my + my. For a particular reaction, the threshold energy might be as shown here: In chemical reactions, the energy barrier corresponds to the amount of energy the particles must have to react when they collide. Only particles that have at least as. The results for the threshold kinetic energy necessary for initiating an endoergic process is given as. In chemical reactions, the energy barrier corresponds to the amount of energy the particles must have to react when they collide. As we have seen, breaking bonds requires energy, and because all reactions involve the breaking of bonds, an input of energy is required to. Two important barriers for effective collisions are an energy barrier (largely enthalpic in nature) and the orientation barrier (mostly. Threshold energy is the minimum amount of energy needed for particles to react.
from 2012books.lardbucket.org
For a particular reaction, the threshold energy might be as shown here: Threshold energy is the minimum amount of energy needed for particles to react. Mx + my + my. The minimum amount of energy required by the colliding molecules to yield the products is called threshold energy (et). As we have seen, breaking bonds requires energy, and because all reactions involve the breaking of bonds, an input of energy is required to. Only particles that have at least as. The results for the threshold kinetic energy necessary for initiating an endoergic process is given as. In chemical reactions, the energy barrier corresponds to the amount of energy the particles must have to react when they collide. Two important barriers for effective collisions are an energy barrier (largely enthalpic in nature) and the orientation barrier (mostly. In chemical reactions, the energy barrier corresponds to the amount of energy the particles must have to react when they collide.
The Collision Model of Chemical
Threshold Energy In Chemical Kinetics Mx + my + my. In chemical reactions, the energy barrier corresponds to the amount of energy the particles must have to react when they collide. Threshold energy is the minimum amount of energy needed for particles to react. Mx + my + my. Two important barriers for effective collisions are an energy barrier (largely enthalpic in nature) and the orientation barrier (mostly. In chemical reactions, the energy barrier corresponds to the amount of energy the particles must have to react when they collide. For a particular reaction, the threshold energy might be as shown here: The results for the threshold kinetic energy necessary for initiating an endoergic process is given as. Only particles that have at least as. The minimum amount of energy required by the colliding molecules to yield the products is called threshold energy (et). As we have seen, breaking bonds requires energy, and because all reactions involve the breaking of bonds, an input of energy is required to.
From www.youtube.com
ACTIVATION ENERGY AND THRESHOLD ENERGY CHCHEMICAL YouTube Threshold Energy In Chemical Kinetics In chemical reactions, the energy barrier corresponds to the amount of energy the particles must have to react when they collide. Two important barriers for effective collisions are an energy barrier (largely enthalpic in nature) and the orientation barrier (mostly. The results for the threshold kinetic energy necessary for initiating an endoergic process is given as. Only particles that have. Threshold Energy In Chemical Kinetics.
From www.youtube.com
Arhenius Equation and Threshold Energy Chemical YouTube Threshold Energy In Chemical Kinetics For a particular reaction, the threshold energy might be as shown here: In chemical reactions, the energy barrier corresponds to the amount of energy the particles must have to react when they collide. Only particles that have at least as. Mx + my + my. Two important barriers for effective collisions are an energy barrier (largely enthalpic in nature) and. Threshold Energy In Chemical Kinetics.
From www.youtube.com
12th chemistry chemical 3 rate law equation, effective Threshold Energy In Chemical Kinetics Only particles that have at least as. As we have seen, breaking bonds requires energy, and because all reactions involve the breaking of bonds, an input of energy is required to. In chemical reactions, the energy barrier corresponds to the amount of energy the particles must have to react when they collide. The minimum amount of energy required by the. Threshold Energy In Chemical Kinetics.
From www.youtube.com
chemical collision theory/ Rate of reactions/ threshold Threshold Energy In Chemical Kinetics Threshold energy is the minimum amount of energy needed for particles to react. Only particles that have at least as. Mx + my + my. As we have seen, breaking bonds requires energy, and because all reactions involve the breaking of bonds, an input of energy is required to. The results for the threshold kinetic energy necessary for initiating an. Threshold Energy In Chemical Kinetics.
From www.youtube.com
25) Activation Energy and Threshold Energy Class12 Chemical Threshold Energy In Chemical Kinetics Two important barriers for effective collisions are an energy barrier (largely enthalpic in nature) and the orientation barrier (mostly. As we have seen, breaking bonds requires energy, and because all reactions involve the breaking of bonds, an input of energy is required to. The minimum amount of energy required by the colliding molecules to yield the products is called threshold. Threshold Energy In Chemical Kinetics.
From fity.club
Chemistry 30 Chemical Activation Energy Threshold Energy In Chemical Kinetics The minimum amount of energy required by the colliding molecules to yield the products is called threshold energy (et). Threshold energy is the minimum amount of energy needed for particles to react. Mx + my + my. For a particular reaction, the threshold energy might be as shown here: As we have seen, breaking bonds requires energy, and because all. Threshold Energy In Chemical Kinetics.
From www.youtube.com
Chemical Intruduction Rate of Reaction, Threshold Energy Threshold Energy In Chemical Kinetics Threshold energy is the minimum amount of energy needed for particles to react. As we have seen, breaking bonds requires energy, and because all reactions involve the breaking of bonds, an input of energy is required to. The results for the threshold kinetic energy necessary for initiating an endoergic process is given as. In chemical reactions, the energy barrier corresponds. Threshold Energy In Chemical Kinetics.
From www.nuclear-power.com
Liquid Drop Model of Nucleus Definition, Facts & Uses Threshold Energy In Chemical Kinetics The results for the threshold kinetic energy necessary for initiating an endoergic process is given as. For a particular reaction, the threshold energy might be as shown here: The minimum amount of energy required by the colliding molecules to yield the products is called threshold energy (et). In chemical reactions, the energy barrier corresponds to the amount of energy the. Threshold Energy In Chemical Kinetics.
From www.expii.com
Rate of Reaction (Enzymes) — Role & Importance Expii Threshold Energy In Chemical Kinetics Two important barriers for effective collisions are an energy barrier (largely enthalpic in nature) and the orientation barrier (mostly. Mx + my + my. For a particular reaction, the threshold energy might be as shown here: Only particles that have at least as. As we have seen, breaking bonds requires energy, and because all reactions involve the breaking of bonds,. Threshold Energy In Chemical Kinetics.
From byjus.com
Reaction Coordinate Diagram An Overview of Reaction Coordinate Threshold Energy In Chemical Kinetics In chemical reactions, the energy barrier corresponds to the amount of energy the particles must have to react when they collide. As we have seen, breaking bonds requires energy, and because all reactions involve the breaking of bonds, an input of energy is required to. Two important barriers for effective collisions are an energy barrier (largely enthalpic in nature) and. Threshold Energy In Chemical Kinetics.
From socratic.org
What is activation energy? What is threshold energy? What are the Threshold Energy In Chemical Kinetics Two important barriers for effective collisions are an energy barrier (largely enthalpic in nature) and the orientation barrier (mostly. Mx + my + my. In chemical reactions, the energy barrier corresponds to the amount of energy the particles must have to react when they collide. Only particles that have at least as. In chemical reactions, the energy barrier corresponds to. Threshold Energy In Chemical Kinetics.
From www.youtube.com
Chemical Activation Energy , Threshold Energy , Enthalpy Threshold Energy In Chemical Kinetics The minimum amount of energy required by the colliding molecules to yield the products is called threshold energy (et). The results for the threshold kinetic energy necessary for initiating an endoergic process is given as. Two important barriers for effective collisions are an energy barrier (largely enthalpic in nature) and the orientation barrier (mostly. As we have seen, breaking bonds. Threshold Energy In Chemical Kinetics.
From www.youtube.com
Chemical p7(activation energy,threshold energy,collision Threshold Energy In Chemical Kinetics In chemical reactions, the energy barrier corresponds to the amount of energy the particles must have to react when they collide. Only particles that have at least as. For a particular reaction, the threshold energy might be as shown here: In chemical reactions, the energy barrier corresponds to the amount of energy the particles must have to react when they. Threshold Energy In Chemical Kinetics.
From www.youtube.com
Chemical 14 Collision theory of chemical reaction Threshold Energy In Chemical Kinetics Threshold energy is the minimum amount of energy needed for particles to react. The results for the threshold kinetic energy necessary for initiating an endoergic process is given as. In chemical reactions, the energy barrier corresponds to the amount of energy the particles must have to react when they collide. Only particles that have at least as. Two important barriers. Threshold Energy In Chemical Kinetics.
From www.youtube.com
Activation energy/threshold energy /activation energy in chemical Threshold Energy In Chemical Kinetics The results for the threshold kinetic energy necessary for initiating an endoergic process is given as. As we have seen, breaking bonds requires energy, and because all reactions involve the breaking of bonds, an input of energy is required to. Only particles that have at least as. For a particular reaction, the threshold energy might be as shown here: Threshold. Threshold Energy In Chemical Kinetics.
From www.youtube.com
Concept of Activation Energy, Threshold Energy Chemistry(12th Threshold Energy In Chemical Kinetics The minimum amount of energy required by the colliding molecules to yield the products is called threshold energy (et). For a particular reaction, the threshold energy might be as shown here: Two important barriers for effective collisions are an energy barrier (largely enthalpic in nature) and the orientation barrier (mostly. As we have seen, breaking bonds requires energy, and because. Threshold Energy In Chemical Kinetics.
From 2012books.lardbucket.org
The Collision Model of Chemical Threshold Energy In Chemical Kinetics Two important barriers for effective collisions are an energy barrier (largely enthalpic in nature) and the orientation barrier (mostly. For a particular reaction, the threshold energy might be as shown here: In chemical reactions, the energy barrier corresponds to the amount of energy the particles must have to react when they collide. Mx + my + my. The minimum amount. Threshold Energy In Chemical Kinetics.
From www.youtube.com
Chemical Rate of Reaction, Threshold Energy, Activation Threshold Energy In Chemical Kinetics The minimum amount of energy required by the colliding molecules to yield the products is called threshold energy (et). In chemical reactions, the energy barrier corresponds to the amount of energy the particles must have to react when they collide. Two important barriers for effective collisions are an energy barrier (largely enthalpic in nature) and the orientation barrier (mostly. For. Threshold Energy In Chemical Kinetics.